

Not all products are available in all regions. GDPR Statement | Declaration for California Compliance Law. Any person depicted in such photographs is a model. Photos displayed are for illustrative purposes only. Your use of this website and the information contained herein is subject to our Website Terms and Conditions and Privacy Policy.
ISTAT BLOOD TEST RESULTS REGISTRATION
The products and information contained herewith may not be accessible in all countries, and Abbott takes no responsibility for such information which may not comply with local country legal process, regulation, registration and usage. This website is governed by applicable U.S. No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to identify the product or services of the company.

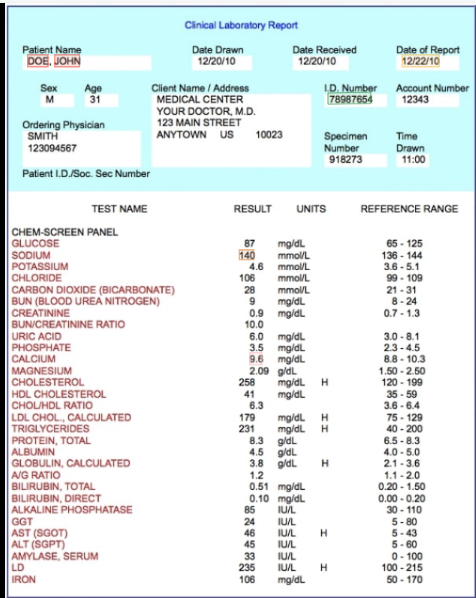
Unless otherwise specified, all product and service names appearing in this Internet site are trademarks owned by or licensed to Abbott, its subsidiaries or affiliates. This page is only accessible to registered i-STAT customers. Reference ranges (sometimes referred to as normal ranges) in the default Customization profile can be found behind login on the i-STAT Cartridge and Test Information (CTI) Sheets/Instructions for Use (IFU) page. Performance characteristics have not yet been established for cTnI values above 35.00 ng/mL Non-heparinized whole blood samples tested within one minute of drawing from a patient into a plastic syringe or plastic evacuated tube containing no additives.Heparinized whole blood or plasma samples collected in syringes or evacuated tubes containing lithium heparin, or.DESCRIPTION: Rapid triage of chest pain patients, including those with ACS.Measurements of cardiac troponin I are used in the diagnosis and treatment of myocardial infarction and as an aid in the risk stratification of patients with acute coronary syndromes with respect to their relative risk of mortality. The i-STAT cardiac troponin I (cTnI) test is an in vitro diagnostic test for the quantitative measurement of cardiac troponin I (cTnI) in whole blood or plasma.


 0 kommentar(er)
0 kommentar(er)
